M13KO7
- P006
- 临界点生物
- 四川省成都市
- 现货
- 1ml
- 2000 元
- 2023-03-09 17:53:22
成都临界点生物科技有限公司
M13KO7
M13KO7 is an M13 derivative, with the origin of replication from P15A and the kanamycin resistance gene from Tn903 both inserted within the M13 origin of replication. M13KO7 is able to replicate in the absence of phagemid DNA. In the presence of a phagemid bearing a wild-type M13 or f1 origin, single-stranded phagemid is packaged preferentially and secreted into the culture medium.This allows easy production of single-stranded phagemid DNA for mutagenesis or sequencing.To propagate (amplify) the M13K07 helper phage requires using the E. coli TG1,ER2738 or strainXL1-Blue MRF′ ,because those strain harbors the supE44 mutation, which provides a glutamine suppressor tRNA.The M13KO7 helper phage is used strictly to produce single stranded DNA from the already excised phagemid. (This is also what VCSM13 is used for.)
Storing the Helper Phage
Recommends storing them at-80℃ with 50% Glycerol. As long as there is a -80℃ stock in the lab, the amplified lab prep can be stored at+4℃.
Titering the Helper Phage
1. Streak out TG1 onto an 2YT plate. Pick a single colony of TG1, inoculate into 2YT and grow to OD600 = 0.6. Meanwhile prepare a serial dilution of the phage in PBS buffer. Expected number of phage should be about 1010 pfu/ml; therefore dilute phage to create dilutions 10-4 to 10-7.
Note:
1.When preparing serial dilutions of bacteriophage, it is usually much more accurate to dilute 10μl into 100 to create a 10-1 dilution or 10μl into 1000μl to create a 10-2 dilution rather than to dilute 1 into 10μl or 1μl into 100μl because one tends to obtain grossly exaggerated titers resulting from small pipetting errors. This is particularly critical for the first dilution.
2.Add 200μl of TG1 cells at OD600 = 0.6 to individual sterile culture tubes (the number of phage dilutions you plan to plate) in a test tube rack.
3.Add 100μl of each serial dilution of helper phage to the culture tubes containing the TG1 cells.
4. Place the test tube rack into a 37℃ water bath for 15 minutes to allow the helper phage to attach to the cells.
5. Meanwhile melt NZY Top Agarose in the microwave and allow it to cool to ~48/50℃.
You need to pay close attention to the bottle containing the top agarose because it can quickly boil over; alternatively use a low power setting on the microwave.
6. At the conclusion of the 15-minute incubation, add ~3 ml of the NZY top agarose to the test tube containing the helper phage & E.coli. Remove the tube from the rack (which is still sitting in the water bath) and give it a quick flick of your wrist mixing the contents and immediately pour it onto an NZY plate. (I usually do three plates at one by pipetting 10 ml of the NZY top agarose from the bottle, dispensing ~3.3 to one tube, ~3.3 to second tube and then another ~3.3 to a third tube. I then pour the plates starting with the first tube I put the NZY into.)
7. Allow the top agarose to cool (~5 minutes).
8. Invert the plate and incubate overnight at 37℃.
9. Count the number of plaques.
Amplifying the Helper Phage
1. Streak out TG1 onto an 2YT plate.
2. Pick a single colony of TG1 and inoculate into 5ml 2×YT and grow until OD600 = 0.3 (~2.5×108 cells/ml).
3. Add helper phage at a multiplicity of infection (MOI) of 20:1 (phage-to-cells ratio).
4. Grow the culture at 37℃ with vigorous aeration (~300rpm) for more 8 hours.
Note: (Add kanamycin to a final concentration of 50μg/ml to the medium 6h after the helper phage and cells have been allowed to grow together.)
5. Add every 1mL of the culture to 100mL 2YT, and culture at 30℃ with 230rpm overnight.
6. Spin culture at 10,000xrpm for 20 minutes. Transfer supernatant to a new tube.
7. Add 1/5 volume of 2.5 M NaCl/20% PEG-8000 solution to tube, and let sit at 4℃ for 2h after mixing.
8. Spin at 10,000xrpm for 20 minutes. Decant the supernatant, and spin with 1mL 1×PBS briefly.
9. Add 1/5 volume of 2.5 M NaCl/20% PEG-8000 solution to tube, and let sit at 4℃ for 1h after mixing.
10. Spin at 10,000xrpm for 20 minutes. Decant the supernatant, and spin with 200uL 1×PBS again briefly.
11.Add 100% glycerin to 50% concentration and store in the refrigerator at -80℃.
VECTOR MAP
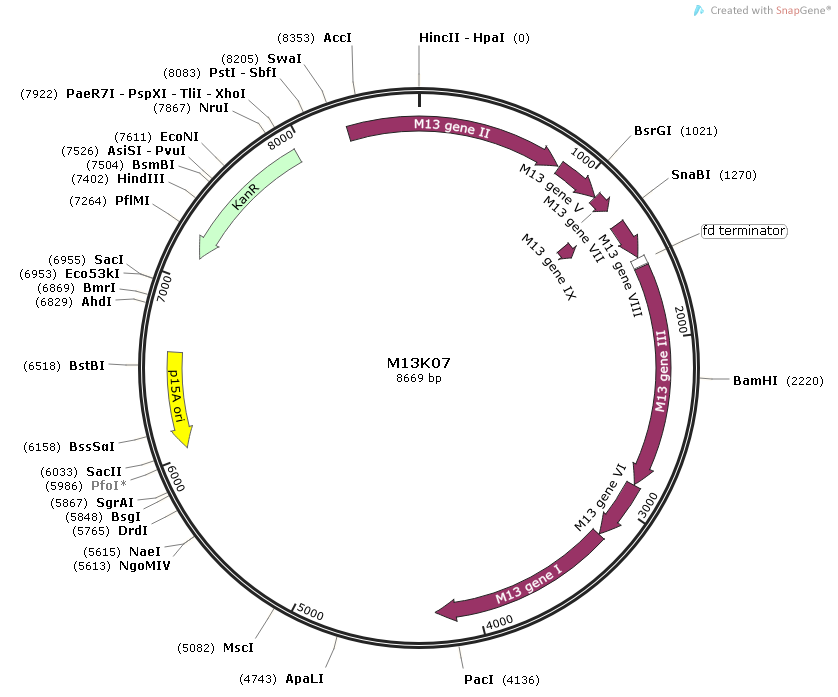
Vector sequence
FASTA