GUCY2C:平衡性的“守护者”,带来结直肠癌免疫治疗新曙光
近年来,GUCY2C在肿瘤免疫治疗中展现出极大的潜力,尤其是CRC CAR-T免疫治疗。
2022年4月19日,上海斯丹赛生物公司宣布,其开发的实体瘤CAR-T产品GCC19CART获得美国(FDA)快速通道资格。GCC19CART是一款基于斯丹赛生物自主研发的实体瘤通用的CoupledCAR®技术平台的CAR-T细胞疗法,通过靶向GUCY2C以治疗复发难治转移型结直肠癌(R/R mCRC) [1]。这款疗法计划于2022年中在美国启动I期临床试验。此前,GCC19CART在中国进行的概念验证(PoC)人体试验中取得了积极的结果。此外,辉瑞的GUCY2C/CD3双特异性抗体,也正在国外做临床前研究 [2]。近年来,GUCY2C在肿瘤免疫治疗中展现出极大的潜力,尤其是CRC CAR-T免疫治疗。那么,GUCY2C是什么?GUCY2C在结直肠癌免疫治疗中的进展如何?一起来了解一下。
1、GUCY2C的结构及其功能
2、GUCY2C的配体
3、GUCY2C介导的信号转导通路
4、GUCY2C在疾病中的作用
5、GUCY2C靶向治疗及临床前景
1、GUCY2C的结构及其功能
鸟苷酸环化酶C (guanylyl cyclase C, GUCY2C/GCC)属于受体鸟苷酸环化酶家族中的一员 [3, 4]。该酶是细菌热稳定肠毒素的肠道受体,所以GUCY2C又称为热稳定肠毒素受体。人GUCY2C基因定位于染色体12q12,其编码产物为一种I型跨膜蛋白,分子量约为120 kDa [5]。GUCY2C蛋白结构主要由5部分组成:①胞外N末端受体结合区。含40%的自身蛋白,可结合特定的配体;②疏水的跨膜区,将胞外信息传入胞内;③胞质区,即激酶同源区,将信号从结合了配体的受体区转至催化区;④催化区;⑤羧基末端 [6, 7] (图1)。大量研究表明,鸟苷酸环化酶C的激活可刺激胞内第二信使--环磷酸鸟苷(cyclic guanosine monophosphate,cGMP)的产生,起到调节体内稳态、维持肠道屏障功能、发挥抗炎活性等作用 [8]。

图1. GUCY2C结构
在正常人机体组织中,GUCY2C主要表达于肠上皮细胞,在肠外组织和肿瘤中不表达。GUCY2C参与的信号转导通路可调节肠道内水和电解质的平衡 [6]。近年来研究发现,GUCY2C在原发性结直肠癌细胞中稳定表达,而在转移性结直肠癌细胞中,GUCY2C异常高表达,被认为是转移性结直肠癌的特异性标志分子 [9]。另有数据表明,GUCY2C在胰腺癌、胃癌和食道癌中表达 [5, 10],提示GUCY2C或可作为这些疾病的潜力靶标。
2、GUCY2C的配体
鸟苷素(guanylin,Gn)和尿鸟苷素(uroguanylin,Ugn)是GUCY2C内源性配体,在人体许多组织器官中都有表达,如肠道、肾、心脏、肺、子宫、卵巢等,其中,在肠道和肾脏的表达尤为丰富 [11]。热稳定性肠毒素(heat-stable enterotoxin,STa)是GUCY2C的外源性配体,由产肠毒素大肠杆菌产生 [12]。如图2所示,Gn和Ugn与细菌STa结构相似,都在肽链中含有丰富的半胱氨酸和二硫键,这是它们发挥生物学功能所必须的结构。其二硫键数量将GUCY2C的配体分成2类:2个二硫键的Gn和Ugn;3个二硫键的STa [7]。半胱氨酸和二硫键数目的不同决定它们生物学功能上的差异 [13]。有数据表明,Gn、Ugn和STa均可抑制肠道对液体和盐的吸收,但抑制能力有明显的不同 [13]。当这些配体(Gn/Ugn/STa)与肠上皮细胞的GUCY2C受体结合后,能激活GUCY2C,使GUCY2C受体具有鸟苷酸环化酶活性,从而发挥生物学效应。此外,Gn、Ugn和STa在蛋白质序列和构象的差异,导致它们同GUCY2C受体结合的亲和力不同,激活GUCY2C受体的能力也不同 [13]。
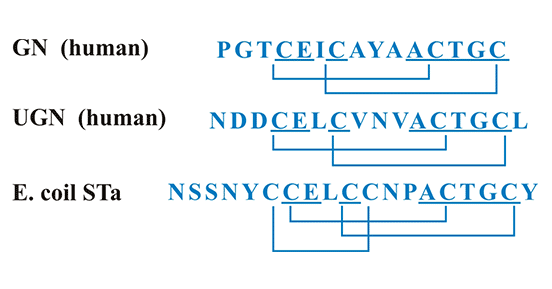
图2. GUCY2C的配体
3、GUCY2C介导的信号转导通路
目前研究表明,GUCY2C通常参与细胞膜离子通道的开启,从而调节细胞内外正常的离子浓度和肠道水电解质平衡,促进肠道的消化吸收,维持肠道功能,保护肠道屏障的完整性。如图3所示,GUCY2C同配体(Gn/Ugn/STa)结合后,GUCY2C激活,介导GTP转化为cGMP,胞内的cGMP浓度增加,导致下游效应器的激活,包括依赖cGMP的蛋白激酶PKGs、磷酸二酯酶PDEs和环核苷酸门控离子(CNG),这一系列信号分子的激活与人体代谢机制密切相关。比如,当GUCY2C诱导的蛋白激酶G II(PKG II)活化时,可磷酸化囊性纤维化跨膜传导调节因子(CFTR)。CFTR经磷酸化后,诱导氯离子等经胞内向胞外流动。有研究表明,当GUCY2C配体缺失时,GUCY2C异常高表达,将导致结直肠上皮细胞稳态失衡,可引起肠道上皮细胞连接形成的肠道屏障被破坏,促进肠道肿瘤的发生,但其具体的调节机制有待进一步阐明 [14]。

图3. GUCY2C介导的信号转导通路
4、GUCY2C在疾病中的作用
GUCY2C受鸟苷素、尿鸟苷素和热稳定性肠毒素的激活,可调节肠道水、电解质的动态平衡和肠上皮细胞转变和增殖。目前研究发现,GUCY2C主要与肠道疾病相关,以及较少报道的神经疾病。其中,肠道类相关的疾病主要包括功能性胃肠病(例如过敏性肠综合症,便秘,或酸度过量等)、炎症性肠病(例如克罗恩病和溃疡性结肠炎等)、癌症(例如胃肠道癌症)。
在肠道疾病方面,有研究提示,GUCY2C的活化突变,可使肠道环境发生变化,从而导致肠道菌群改变并增加克罗恩病的易感性 [15]。另有报道,GUCY2C与先天性失钠性腹泻有关,且家族性GUCY2C腹泻综合症可在成年后发展为炎症性肠病 [16]。在神经系统疾病方面,有研究表明GUCY2C选择性地表达于中脑多巴胺神经元上,并以独特的生理机制调控动物的注意力和运动水平 [17]。
在胃肠道肿瘤方面,GUCY2C在大肠癌患者外周血中具有较高的阳性表达率,提示GUCY2C在大肠癌复发或转移病例中,可作为患者术后复发和转移的早期检测指标 [18]。另有研究表明,GUCY2C在胃黏膜肠上皮化生、异型增生及胃癌中高表达 [19]。也有报道,GUCY2C是一种区分原发性和转移性卵巢黏液瘤较特异的标志物 [20]。值得关注的是,大量研究提示,GUCY2C在结肠癌变的早期阶段,常出现表达异常 [21, 22]。有研究数据表明,在转移性结直肠癌细胞中,GUCY2C特异性高表达,其作为结直肠癌的分子标志物潜力巨大 [23]。
5、GUCY2C靶向治疗及临床前景
GUCY2C作为CAR-T近期热门研究靶点,国内上海斯丹赛生物技术有限公司的GUCY2C CAR-T 细胞疗法刚被授予FDA快速通道资格,有望很快在美国进行I期临床。事实上,已有早期临床试验表明,在甲状腺癌或结肠直肠癌患者中,GUCY2C CAR-T治疗显著抑制了肿瘤生长,其疗效显著。基于GUCY2C的免疫治疗,早前辉瑞的GUCY2C/CD3双特异性抗体PF-07062119临床前研究结果就已证实,GUCY2C在结直肠癌(CRC)中的抗肿瘤活性,且毒性可控。此外,PF-07062119与抗PD-1/PD-L1治疗联合使用,可增强抗肿瘤活性。另有报道,美国的托马斯杰斐逊大学和Sidney Kimmel癌症中心科研团队开发了专门针对结直肠癌的疫苗Ad5-GUCY2C-PADRE,将GUCY2C分子与增强免疫反应的分子PADRE连接起来,并将其加载到腺病毒载体中。临床前研究表明,该疫苗可激活CD8+T细胞产生免疫应答,预防结直肠癌转移 [24]。
不管是疫苗或CAR-T细胞等免疫疗法,新疗法的出现必将给结直肠癌在内的胃肠道恶性肿瘤的治疗带来新的希望。GUCY2C作为肠道信号系统中的重要一员,以GUCY2C为作用靶点的研究强调了GUCY2C在结直肠癌中扮演的重要角色,而将GUCY2C CAR-T或疫苗应用到结直肠癌治疗更是重大突破,这对于CRC患者的治疗将具有重大意义。
为鼎力协助各药企针对GUCY2C在结肠癌等肠道类疾病的药物研发工作,CUSABIO推出GUCY2C活性蛋白产品(Code: CSB-MP010053HUd9;Code: CSB-MP010053HU),助力您在GUCY2C机制方面的研究或其潜在临床价值的探索。
● Recombinant Human Heat-stable enterotoxin receptor (GUCY2C),partial (Active)
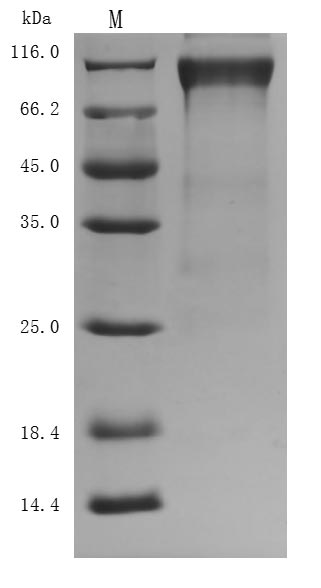
The purity was greater than 94.8% as determined by SDS-PAGE. (Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
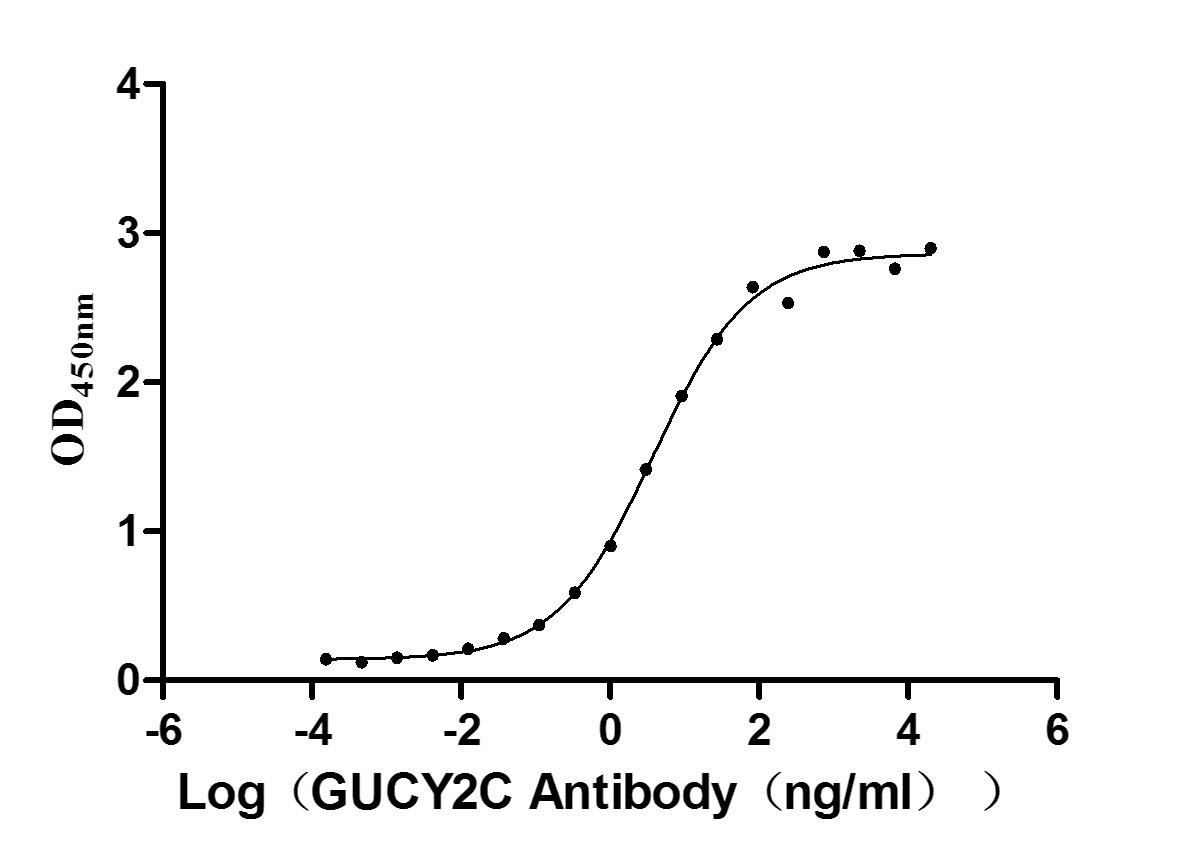
Immobilized human GUCY2C at 5 μg/ml can bind Anti-GUCY2C recombinant antibody (CSB-RA010053A2HU), the EC50 is 3.049-4.660 ng/mL.
● Recombinant Human Heat-stable enterotoxin receptor (GUCY2C),partial (Active)

The purity was greater than 95% as determined by SDS-PAGE. (Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.

Immobilized human GUCY2C at 5 μg/ml can bind Anti-GUCY2C recombinant antibody (CSB-RA010053A2HU), the EC50 is 11.89-24.13 ng/mL.
参考文献:
[1] Chen, Naifei, et al. "Dose Escalation Study of GCC19CART CoupledCAR® Technology for Patients with Relapsed or Refractory Colorectal Cancer." Blood 138.Supplement 1 (2021): 4841-4841.
[2] Maresca, Kevin P., et al. "Preclinical Evaluation of 89Zr-Df-IAB22M2C PET as an Imaging Biomarker for the Development of the GUCY2C-CD3 Bispecific PF-07062119 as a T Cell Engaging Therapy." Molecular Imaging and Biology 23.6 (2021): 941-951.
[3] Snook, Adam. "GUCY2C‐Directed CAR‐T Cell Therapy for Upper‐GI Cancers." The FASEB Journal 35 (2021).
[4] Pattison, Amanda M., et al. "Silencing the intestinal GUCY2C tumor suppressor axis requires APC loss of heterozygosity." Cancer biology & therapy 21.9 (2020): 799-805.
[5] Flickinger Jr, John C., et al. "Guanylyl cyclase C as a biomarker for immunotherapies for the treatment of gastrointestinal malignancies." Biomarkers in Medicine 15.3 (2021): 201-217.
[6] Hasegawa M, Hidaka Y, Matsumoto Y, Sanni T, Shimonishi Y. Determination of the binding site on the extracellular domain of guanylyl cyclase C to heat-stable enterotoxin. J Biol Chem. 1999 Oct 29;274(44):31713-8.
[7] Potter, Lincoln R. "Domain analysis of human transmembrane guanylyl cyclase receptors: implications for regulation." Front Biosci 10.1 (2005): 1205-1220.
[8] Blomain, Erik S., et al. "APC-β-catenin-TCF signaling silences the intestinal guanylin-GUCY2C tumor suppressor axis." Cancer biology & therapy 21.5 (2020): 441-451.
[9] Jimenez-Luna, Cristina, et al. "Circulating PTGS2, JAG1, GUCY2C and PGF mRNA in Peripheral Blood and Serum as Potential Biomarkers for Patients with Metastatic Colon Cancer." Journal of clinical medicine 10.11 (2021): 2248.
[10] Wilson, Chantell, et al. "The paracrine hormone for the GUCY2C tumor suppressor, guanylin, is universally lost in colorectal cancer." Cancer Epidemiology and Prevention Biomarkers 23.11 (2014): 2328-2337.
[11] Lisby, Amanda N., et al. "GUCY2C as a biomarker to target precision therapies for patients with colorectal cancer." Expert Review of Precision Medicine and Drug Development 6.2 (2021): 117-129.
[12] Romi, Hila, et al. "Meconium ileus caused by mutations in GUCY2C, encoding the CFTR-activating guanylate cyclase 2C." The American Journal of Human Genetics 90.5 (2012): 893-899.
[13] GU, Xuemei, Lei FENG, and Maode LAI. "Research progress in guanylin family." Chinese Journal of Pathophysiology (2000).
[14] Rappaport, Jeffrey A., and Scott A. Waldman. "The guanylate cyclase C—cGMP signaling axis opposes intestinal epithelial injury and neoplasia." Frontiers in oncology 8 (2018): 299.
[15] Tronstad, Rune R., et al. "Guanylate cyclase C activation shapes the intestinal microbiota in patients with familial diarrhea and increased susceptibility for Crohn's disease." Inflammatory bowel diseases 23.10 (2017): 1752-1761.
[16] Nambu, Ryusuke, et al. "A Systematic Review of Monogenic Inflammatory Bowel Disease." Clinical Gastroenterology and Hepatology (2021).
[17] Liu, Lu, et al. "Association between GUC2C and ADHD: Evidence from both categorical and quantitative traits." Psychiatry research 220.1-2 (2014): 708-710.
[18] Bashir, Babar, et al. "Silencing the GUCA2A-GUCY2C tumor suppressor axis in CIN, serrated, and MSI colorectal neoplasia." Human pathology 87 (2019): 103-114.
[19] Snook, Adam E., Michael S. Magee, and Scott A. Waldman. "GUCY2C-targeted cancer immunotherapy: past, present and future." Immunologic research 51.2 (2011): 161-169.
[20] Ciocca, Vincenzo, et al. "Guanylyl cyclase C is a specific marker for differentiating primary and metastatic ovarian mucinous neoplasms." Histopathology 55.2 (2009): 182-188.
[21] Waldman, Scott A., and Michael Camilleri. "Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders." Gut 67.8 (2018): 1543-1552.
[22] Aka, Allison A., et al. "Guanylate cyclase C as a target for prevention, detection, and therapy in colorectal cancer." Expert review of clinical pharmacology 10.5 (2017): 549-557.
[23] Pitari, G. M., et al. "Bacterial enterotoxins are associated with resistance to colon cancer." Proceedings of the National Academy of Sciences 100.5 (2003): 2695-2699.
[24] Snook, Adam E., et al. "Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced T-cell responses in colon cancer patients." Journal for immunotherapy of cancer 7.1 (2019): 1-12.
咨询
- 523
- 点赞
- 复制链接
- 举报


